Medical Injection Molding
Medical injection molding companies may all offer the same service but only ICOMold by Fathom can put its state-of-the-art plastic injection molding facilities at your disposal. Medical plastic injection molding requires a level of expertise that only comes with decades of experience. ICOMold is an ISO 9001:2015 certified and has been providing low cost injection molding to a variety of industries since 2003.
What is Medical Injection Molding?
Injection molding is a fast and cost-effective manufacturing process used to make plastic parts in large quantities. Medical injection molding is the process of making these plastic parts for use in the medical industry.
Injection molding starts with a steel mold. A negative cavity of the finished part is machined into two pieces of high-strength steel. The two pieces of the mold are then placed in the injection-molding machine and held together under pressure. Molten plastic is then injected into the mold. The mold opens once the plastic has solidified and the part is ejected before the process starts over.
Not all medical injection molding companies are the same. ICOMold by Fathom is an ISO 901:2015-certified company and has experience making products for a variety of industries.
FDA Requirements
The Federal Food and Drug Administration (FDA) has different requirements for plastic materials based on the amount of contact the device or part will have with the human body. These requirements are divided into three broad categories.
- The most regulated plastic in medical devices are those that will be implanted in the human body. These medical devices have a long list of requirements and must be made of certified non-toxic materials and materials proven not to break down over the lifetime of the implant. These parts must also be manufactured in a certified clean room.
- The next category of medical devices are rated for prolonged, external skin contact with the human body. These medical devices have more relaxed FDA requirements and can be manufactured by most injection molding companies.
- The last category of medical devices will have limited contact with the human body. An example would be a cardiac monitor. Human interaction is limited to interacting with the plastic outer shell or the plastic buttons of the monitor. This category of medical devices has the fewest FDA regulations when compared to the other medical device categories.
Selecting The Proper Medical Grade Plastic Materials
There are many different grades of plastic that can be used in medical plastic injection molding. Each type of plastic has a set of strengths and weaknesses to consider. All of the factors below must be considered when planning for medical plastic injection molding.
Strength
The plastic material of choice must have the proper strength. Plastic resins all have differing durability and it is important to choose one that fits the specifications of the medical device. Choosing one that is not strong or durable enough may lead to failure of the medical device.
Resistance to Chemicals and Heat
The plastic material of choice might need to be resistant to heat and chemicals. Knowing where and how a device will be used is important before injection molding medical devices. The plastic material must be able to withstand high temperatures if the injection molded medical device will be subject to standard sterilization methods. The plastic material must be durable and non-degradable if it will have to endure harsh cleaning methods. Cleaning and sterilization methods include high-heat and water pressure, radiation, chemicals, and high-intensity ultra violet light. Failing to take these conditions into account can reduce the life of the injection molded medical device.
Anti-Microbial
Anti-microbial additives are available for many plastic resins. Untreated plastic will support the growth of fungus, algae, and other microbes. This can lead to unsightly discoloration, unpleasant odor, and plastic degradation. Anti-microbial additives can be added during the medical device injection molding process. This can reduce or inhibit microbial growth when a microbe lands on the surface of the device. An injection molded medical device that promotes microbial growth will require more frequent sanitization, which can shorten the life of the device.
Operating environment
The medical-grade plastic material will ultimately be determined by the conditions that the medical device will be exposed to on a daily basis. Some conditions that need to be considered are chemical resistance, corrosion resistance, and exposure to radiation and extreme temperature. Failing to take the operating environment into consideration can cause faster degradation or complete failure of the injection molded medical device.
The experts at ICOMold can help you choose the right plastic material for your next project.
Medical Grade Plastic Materials
The medical grade plastic material used for injection molding is an important choice and should be thoroughly researched. Choosing the wrong plastic material for medical device injection molding could result in a customer paying more for features they do not need, or worse yet, the device fails because the material was not suited for the application. ICOMold carries several medical-grade plastic materials, including:
Polycarbonate
This medical-grade plastic has high-shear, tensile, and flexural strength. Polycarbonate is stronger than acrylic. This material absorbs very little moisture and has high resistance to heat. Polycarbonate is naturally transparent and widely used in medical tubing.
Polyamide (Nylon)
Nylon is widely used due to its strength, toughness, and chemical and wear resistance. Nylon can withstand a wide array of chemicals but is susceptible to strong acids and alcohol. Nylon has excellent heat stability and can be used in environments with temperatures up to 185°C (365°F).
Questions To Ask Before Selecting Plastic When Injection Molding Medical Devices.
Will the plastic material work with the design?
The medical-grade plastic selected for the project must have sufficient tensile strength in relation to the design of the part. If the design calls for thin walls, the plastic material must have enough tensile strength to keep the part from failing. Understanding how materials behave is important in optimizing the medical device for functionality, performance, and durability.
What environmental factors will affect the medical device?
Will the device come into contact with bodily fluids, harsh chemicals, high-temperature sanitation, or other elements? Determining the operating environment of the medical device will help you choose the best plastic material for the application.
What physical forces will the device encounter?
Will the medical device require a certain degree of tensile strength, elasticity, or hardness? Consider conducting finite element analysis (FEA) testing on any design before entering production. FEA uses a computer model of the device to predict how the product will react to certain environmental forces such as vibration, impact, heat, or fluids. FEA tests will show potential points of failure in the design where the device may wear down quickly, twist, bend, or break. Some FEA software databases or companies may already have information on many of the stress and strain specifications of certain plastic materials.
Get a Medical Injection Mold Quote
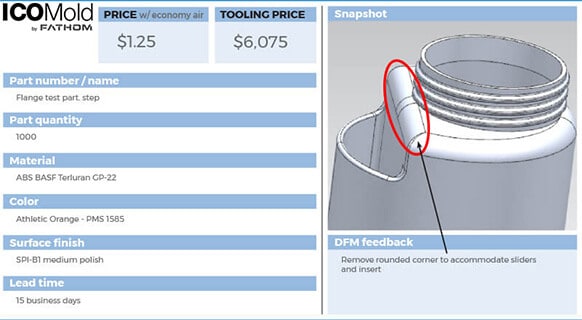
Getting a quote for injection molding medical devices is easy. The first step is to register an account and upload a CAD file. Once the CAD file has been submitted, our instant quote system will evaluate the design and give you an estimate. ICOMold engineers will review the CAD file and confirm the quote. The manufacturing process will begin once the quote has been accepted and confirmed. The time between submitting your CAD file and receiving your first part can be as soon as 15 days.
Medical Injection Molding Applications
There are various applications for medical device injection molding. These applications include:
- Emergency Room Instruments
- Wearable Medical Devices
- Surgical Implants
- Drug Delivery Systems
- Women’s Healthcare Products
- Surgical Devices and Instruments
How Long Does a Medical Injection Mold Last?
Medical plastic injection molds from ICOMold by Fathom will typically last well over 100,000 cycles. ICOMold provides a dedicated project manager and engineer for every injection molding production. Please refer to ICOMold’s Online Project Management System for more information on project timelines.
Post-Processing Options
ICOMold by Fathom offers a wide array of post-processing services. Secondary processes include:
- Post-Molding Machining: Additional CNC machining is completed on injected parts. This process can be used to remove flash or finish the product to meet design specifications.
- Silk Screening and Pad Printing: Detail and branding can be added to mold injected parts. This includes printing custom color graphics for logos, labeling, or part number identification.
- Paint: Spray painting is the simplest and most cost-effective way to add color to parts. The experts at ICOMold can suggest the paint best suited to the part’s final application.
- Powder Coating: Powder coating is the process of spraying plastic powder onto parts. Ultraviolet light is then used to adhere the coating to the plastic part. Powder coating is more durable than paint and adds an extra layer of protection against impacts, moisture, and chemicals. Powder coating is available in a variety of colors and textures.
- Assembly: ICOMold can assemble the parts into a finished product.
- Packaging: After assembly, ICOMold can package completed items into clamshells, blister packs, or boxes. Your product will be packaged and ready for distribution upon arrival.
Injection Molding Production Highlights
> Instant mold and part quote
> Low cost, quick build and quality production
> Online project management
> Trouble-free part modifications
> No size limitations
> Any commercially available material and surface finish
What is the process for medical injection molding production?
ICOMold’s instant online plastic injection molding quote and mold frame sharing technology enables us to simplify and shorten both the quoting and tooling manufacturing process for custom plastic injection molding.
- Load your 3D CAD file to get an instant mold and part quote
- Upon order confirmation, ICOMold starts the mold and part order process
- Tooling design review by ICOMold engineers
- Upon design approval, ICOMold begins building your injection mold
- Customer examines samples for approval
- Part production begins
Go to our plastic injection molding and CNC machining case studies to see how we helped customers with their projects.